You’ve Smelt It but Never Heard of It: Meet d-Limonene
D-Limonene is one of the most used ingredients in perfume and fragrance. Yet, the majority of people have not even heard of it. This article provides some insights into what d-limonene is, and how it's conquering the market.
THE SCENT DAILY
6/6/20255 min read
Open some diffuser blend labeled Citrus Sunrise and the first thing you notice is an upbeat, almost sparkling scent. Nine times out of ten the molecule responsible is d-limonene. In sweet-orange oil it can top 90 % of the total volatiles, making limonene the “biggest little ingredient” in modern home fragrance.
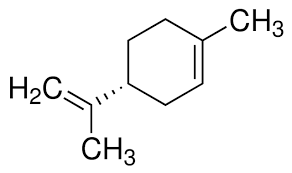
Limonene (C₁₀H₁₆) is a monocyclic monoterpene that exists as two mirror-image forms d-limonene and l-limonene. Chemists refer to this pair of mirror-image forms as enantiomers. They exist as isomers as one form of limonene will rotate the plane of incoming polarized light in the opposite direction of the other. Basically, in layman terms, you can imagine them as a pair of gloves. The right-hand gloves mirrors the left-hand glove, but when you try to place both gloves on top of each other, they are actually different.


As seen in the diagram of the gloves, d-limonene can be thought of as the right-handed glove. While l-limonene can be thought of as the left handed glove. You see how they are actually different? This slight different in the chemical world causes a vast change in what the our brain perceives the chemical to smell like.
D-limonene smells unmistakably of citrus; its sibling l-limonene leans piney and turpentine-like. Clearly, the former has more demand in the world of perfumes and fragrance.
How is this miracle compound produced?
Limonene is mainly produced by citrus plants. However, plants unsurprisingly don't produce it for pleasure. Citrus fauna actually weaponize limonene as a kind of molecular “no-trespassing” sign. Many sap-sucking insects read its sharp volatility as danger—thin, oily vapors that gum up their respiratory spiracles and causes them to fly away. Evolution fine-tuned their antennae to recoil at even trace amounts, much the way we humans flinch at ammonia.
Yet our brains filed the same molecule under pleasant. Neurologists think that divergence comes down to which smell receptors dominate each species’ survival playbook. This makes sense. Humans are hunting animals, but we have been gatherers even longer. Humans, in the past, have sought out fruit for nutrition and thus natural selection has trained us to have a liking for the smell of fruits, especially citrus ones rich in vitamins and nutrients.
Industry has leaned into this evolutionary quirk. Juice factories once paid to haul away mountains of sticky peels as trash; now they spin them in cold-press extractors and sell the resulting orange oil by the railcar. Because that oil tests at up to 97 % d-limonene, a single day’s juice run can furnish enough solvent to scent floor cleaners across an entire continent. When you mop the kitchen and the air blooms citrus-fresh, you’re inhaling the very compound that keeps fruit flies and other insect pests away.
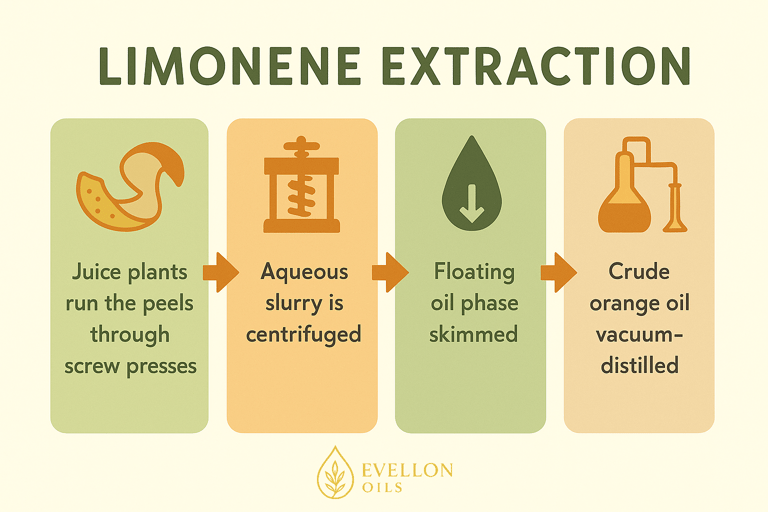
The industrial route is refreshingly low-tech. Juice plants run the peels through screw presses; the aqueous slurry is centrifuged, and the floating oil phase is skimmed. Crude orange oil is then vacuum-distilled to concentrate limonene or—if a “natural” claim is important—left as-is. The carbon footprint is small compared with petroleum-derived solvents, because the feedstock is literally food waste. That’s one reason sustainability-minded brands love to highlight limonene on ingredient panels.
Why Use Limonene?
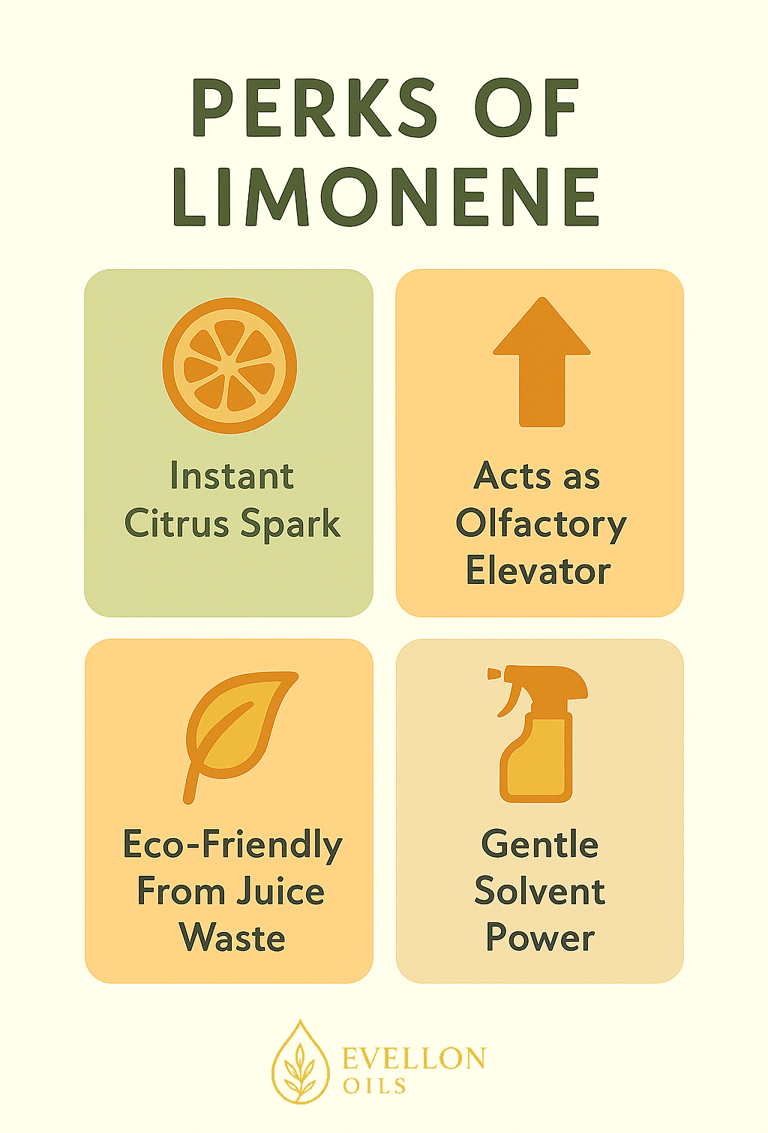
Step into any aisle devoted to candles, plug-ins, or reed diffusers and you’re almost guaranteed to meet d-limonene before you finish the first sniff test. Perfumers prize the molecule for two complementary reasons. First, it volatilizes early and cleanly: with a boiling point around 176 °C and minimal polarity, limonene flashes off the reeds as soon as it reaches room temperature, giving consumers that instant “wow” of freshly peeled citrus. Second, its relatively high vapor pressure creates a convection effect—sometimes called lift—that shepherds slower, heavier molecules out of the carrier solvent and into the air stream. So, heavier molecule fragrances like sandalwood and tonka components can be carried up and diffuse better throughout the space.
Because limonene is both abundant and inexpensive, formulators treat it almost like a functional raw material rather than a luxury note. A typical reed-diffuser accord might open with 30–40 % d-limonene blended with smaller doses of citral, β-pinene, or litsea cubeba to widen the sparkle. Left alone, that proportion would yield a burst of orange zest that fades within a few hours and risks smelling “pithy” as oxidation products accumulate. To moderate the curve, perfumers add fixatives and resinous modifiers—benzoin resinoid, amyris, and even trace vanillin—molecules with lower vapor pressures that form a slow-evaporating lattice. The limonene still evaporates first, but its departure drags a microscopic film of these heavier molecules along.
Limonene is also highly-functional for other purposes. Aerosol sprays may carry upward of 70 % to function as both solvent and scent, dissolving terpene-based oils that would otherwise separate from water. Gel fresheners encapsulate limonene in a porous polymer matrix; as the gel dehydrates, limonene migrates to the surface and volatilizes in controlled pulses. Even soy-wax candles rely on limonene’s heat-triggered release to push a “throw” of fragrance beyond the coffee table.
Outside of fragrance, d-limonene is a champion degreaser. It dissolves adhesives, strips paint and lifts motor-oil stains with a pleasant scent instead of the chemical burn of xylene. The FDA permits food-grade limonene as a flavoring, and researchers have explored it as a “chemo-preventive” agent in animal cancer models. Clearly, it's hyper-versatility in function paired with its easiness to produce has made limonene one of the main foundation for compounds involved in fragrance and cleaning products.
The Booming Market for Limonene
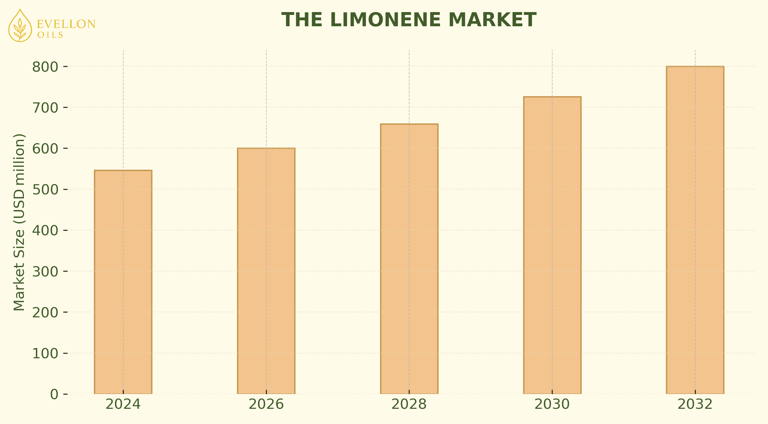
Consultancies that track flavor and fragrance chemicals valued the global limonene market at about USD 546 million in 2024. Forecasts converge on USD 800-820 million by 2032, implying a compound annual growth rate just under 5 %. Two main macro forces engender this trend:
The “natural swap” in household chemicals. Regulatory pressure on VOCs and consumer backlash against petro-derived solvents have driven formulators toward renewable terpenes. Limonene replaces n-paraffins in degreasers, xylene in paint strippers, and acetone in adhesive removers—often improving odor profile while meeting green-label criteria set by big-box retailers. Each new cleaning SKU that adds “citrus-based solvent” to its label locks in several tones of annual demand. Consumers also love the "natural" label, and with d-limonene, large firms can now use that label to please consumers more, and even charge more.
The increasing orange-juice demand. The UN’s Food and Agriculture Organization estimates global orange-juice consumption is growing at around 2 % per year, driven by rising middle-class incomes in Asia-Pacific and steady per-capita intake in North America and Europe. Because limonene yield is tied linearly to peel volume, supply scales effortlessly with that appetite. Even weather-related crop swings in Brazil or Florida tend to be cushioned by frozen-concentrate inventories, keeping feedstock prices stable even in times when they shouldn't.
Bottom line, as long as the world keeps craving orange juice and consumers keep chasing “naturals,” limonene will stay one of the cheapest, greenest, and most scalable aroma molecules in the global fragrance arsenal.