The Role of Alcohol in Perfume Formulas
Why most perfumes contain alcohol, what types are used, and how it affects both evaporation and scent delivery on skin.
THE SCENT DAILY
6/30/20257 min read


Perfumes captivate our senses. It has the ability to evoke memories, emotions, and even transform moods at times. While fragrances are composed of carefully balanced aromatic compounds, one common yet often overlooked ingredient plays a pivotal role: alcohol. Its inclusion is not merely a filler or diluent; alcohol significantly influences how perfumes behave, evolve, and deliver their scent when applied to the skin. In this article, we explore why alcohol is essential in perfume formulas, the types used, and how it shapes scent delivery.
Why Alcohol is in Perfumes
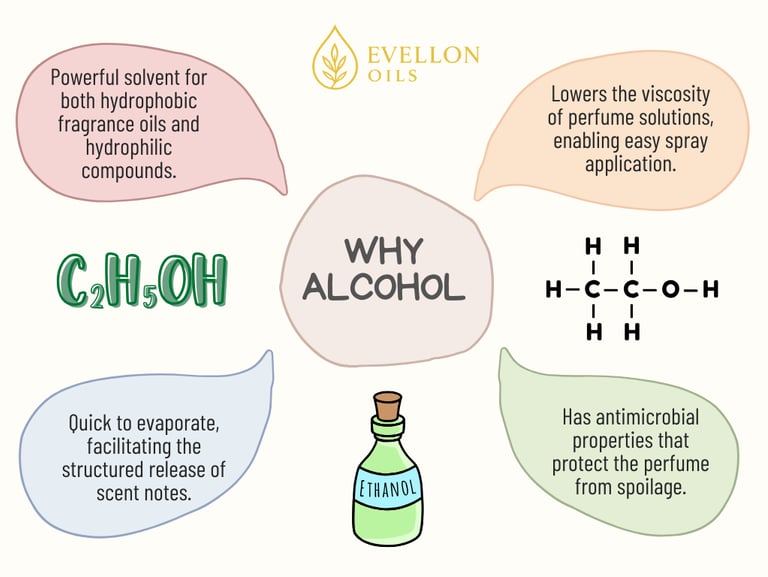
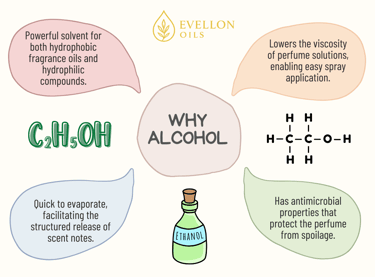
Alcohol serves multiple critical purposes within perfume compositions. At its simplest, it acts as a solvent. Alcohol dissolves aromatic oils and ensures they mix uniformly. Why? Because fragrance oils are hydrophobic, meaning they do not mix well with water, but alcohol, especially ethanol, possesses both hydrophilic (water-attracting) and lipophilic (oil-attracting) characteristics. This nature enables it to effectively dissolve both water-soluble and oil-soluble ingredients, making it an ideal medium for blending complex fragrance compositions.
Without alcohol, perfumes would be oily, thick, and difficult to spray or disperse evenly. Alcohol reduces the viscosity of the fragrance oil mixture, allowing it to be delivered as a fine mist through spray mechanisms. This property allows the perfume to spread uniformly over the skin or clothing.
Additionally, alcohol aids in the preservation and stability of perfume formulations. Ethanol is inherently antimicrobial, meaning it inhibits the growth of bacteria and fungi. This antimicrobial property extends the shelf life of perfumes, protecting them from spoilage and maintaining their aroma over time. It also eliminates the need for additional preservatives in most cases.
Alcohol also plays a pivotal role in fragrance delivery by affecting the evaporation rate. Ethanol has a low boiling point (78.37°C), meaning it evaporates quickly when exposed to air and body heat. This rapid evaporation helps release the perfume’s volatile aromatic molecules into the air. As the alcohol evaporates, it carries the lighter molecules—the top notes—into the air first. These top notes form the initial impression of a fragrance. As more alcohol dissipates, the middle and base notes—comprising heavier, slower-evaporating molecules—become prominent. This sequential unfolding of scent layers is essential to the structured olfactory journey that perfume designers create.
Types of Alcohol Commonly Used
Not all alcohols are suitable for perfumery. The most commonly used alcohol is ethanol (also known as ethyl alcohol or grain alcohol), specifically denatured ethanol. While pure ethanol is chemically identical to the kind found in alcoholic beverages, the version used in perfumes is not meant for consumption. "Denatured" means that specific substances—called denaturants—are added to the ethanol to make it intentionally undrinkable or toxic. These additives may include methanol, isopropyl alcohol, acetone, or bittering agents.
This process of denaturing ethanol is done to comply with regulatory and taxation requirements. Pure ethanol, being potable, is subject to beverage alcohol taxes. However, by adding denaturants and making the ethanol unfit for human consumption, manufacturers can avoid those high taxes levied on consumable alcohol. It also prevents abuse or diversion of industrial or perfumery alcohol for illicit drinking purposes. We don’t want people buying fragrance and chugging them down to get drunk.
Governments and regulatory agencies, such as the U.S. Alcohol and Tobacco Tax and Trade Bureau (TTB) or the EU REACH regulation, require denaturing of ethanol used in non-food applications to control public health risks and prevent revenue loss from misappropriated alcohol. Basically, denaturing ethanol allows perfumers to legally and economically use high-purity alcohol in fragrances without violating such controls.
The ethanol used in perfumery must be nearly odorless to avoid interfering with the aromatic profile of the fragrance. High-quality perfumery ethanol typically has purity levels above 95%. This makes sure that it evaporates cleanly and allows the true scent of the fragrance oils to shine through.
Another alcohol sometimes used in fragrance formulations, though less commonly, is isopropyl alcohol (IPA). However, IPA has a more noticeable, sharp medicinal odor and evaporates differently. These characteristics can disrupt the intended scent of the perfume or change how it unfolds on the skin. As a result, ethanol remains the preferred and most effective choice for the majority of fragrance applications.
How Alcohol Affects Evaporation and Fragrance Dynamics
Perfume unfolds in stages known as notes: top, middle (or heart), and base notes. These stages correspond to different evaporation rates, and alcohol is the medium that enables this layering to happen in a controlled and predictable way.
When you spray perfume, the alcohol indeed evaporates rapidly—it’s designed to. But what's crucial is how it acts as a carrier for the fragrance molecules. Not all fragrance ingredients are equally volatile. Some molecules are light and evaporate quickly (top notes), while others are heavier and slower to evaporate (base notes). Alcohol helps release each of these molecules according to their individual volatility.
At first glance, it may seem like all the fragrance compounds are just blended together and should evaporate at the same time. But perfumers prevent this by carefully designing the formula. They deliberately choose and balance fragrance ingredients based on their molecular weight and volatility. The most volatile compounds—the top notes—are present in greater proportion relative to their strength and are the first to be lifted by the evaporating alcohol. The heavier, less volatile compounds—heart and base notes—are designed to linger because their molecular structures require more time and warmth to evaporate.
So while the alcohol itself does evaporate first, it does so in stages, carrying different fragrance molecules into the air depending on how easily those molecules evaporate. The most volatile ones—top notes—evaporate and disperse first, giving the perfume its bright, sharp opening. As the alcohol continues to evaporate and the concentration of the lighter molecules in the solution diminishes, the less volatile molecules that make up the heart and base notes start to become more prominent. It is often misunderstood that this progression is about the alcohol "thinning out" and thus evaporating slower. But this is not true. The evaporation rate of the alcohol is about constant. Instead, it is about how it gradually stops carrying the lighter molecules (because they’ve already evaporated) and begins releasing the heavier ones that require more time and warmth to lift into the air.
This natural volatility gradient, combined with alcohol’s evaporative power, is what allows perfumers to design fragrances that unfold in layers over time. Without alcohol acting as this structured delivery system, all the fragrance molecules would be released too quickly or at once, and the elegant development of a perfume—from top to heart to base—would be lost.
Alcohol and Skin Chemistry
Beyond evaporation, alcohol also plays a crucial role in scent delivery by interacting directly with skin chemistry. Ethanol is a volatile substance, and as it evaporates, it draws moisture from the surface of the skin—a process called trans-epidermal water loss. This moisture-stripping effect can leave the skin feeling dry, especially with frequent or high-concentration use. For individuals with sensitive or already dry skin, this can lead to irritation or a feeling of tightness.
Additionally, alcohol can affect the skin barrier. While it doesn’t literally open pores like a steam treatment would, ethanol can slightly disrupt the lipid barrier in the uppermost layer of the skin (the stratum corneum). This disruption makes the skin more permeable for a short time, allowing some aromatic compounds to interact more closely with the skin’s surface oils and proteins. This is why fragrance can seem to "settle" into the skin and evolve uniquely for different individuals as it's literally reacting with their personal chemistry.
To counteract the drying effects of alcohol and better serve those with sensitive skin, perfume makers may use alternative solvents in alcohol-free fragrances. These include ingredients like vegetable glycerin, jojoba oil, or fractionated coconut oil. These substances are non-volatile, meaning they do not evaporate quickly like alcohol. Instead, they sit on the skin and gradually release the fragrance. They also act as emollients, helping to moisturize and soften the skin. This not only avoids the dryness associated with alcohol but can also result in a different scent experience. Often, it is closer to the skin, longer-lasting, and more intimate, though typically less diffusive in the air.
Evolution of Alcohol-Free Fragrances
In recent years, consumer preferences have shifted away from alcohol. This shift has been driven by a growing awareness of skin sensitivity, a broader movement toward natural and gentle ingredients, and a demand for clean beauty products. Some consumers have concerns about how alcohol feels on their skin—especially those with dry, eczema-prone, or reactive skin types. Others are motivated by lifestyle preferences, such as avoiding synthetic chemicals or seeking formulations that align with holistic or organic values.
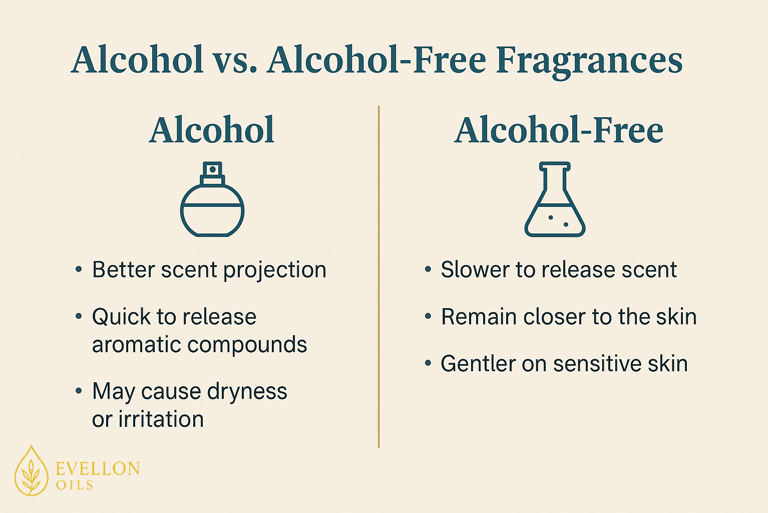
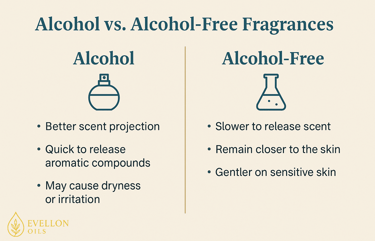
Alcohol-free fragrances function differently but can still deliver a rich olfactory experience. Instead of relying on ethanol to carry and release aromatic compounds into the air, these fragrances use carriers like water, glycerin, or natural oils. The fragrance compounds are suspended or emulsified within these bases. When applied, they release scent more slowly and remain closer to the skin. This means they don’t have the same burst of projection as alcohol-based perfumes, but they often last longer in close proximity which has its own pros and cons.
It’s important to note that alcohol in perfumes is not harmful to health when used externally and in moderation. Ethanol used in perfumery is denatured to prevent ingestion, but it is safe for topical use. The concerns around alcohol are typically about dryness or irritation, not toxicity. For the vast majority of users, alcohol-based perfumes are perfectly safe. Nonetheless, those with allergies, very sensitive skin, or certain medical conditions may prefer to avoid alcohol for comfort and peace of mind.
Despite the rise of alcohol-free options, alcohol remains the dominant choice due to its superior scent dispersion, clarity, and performance. Its ability to lift and project top notes really is unmatched.
Quality and Concentration of Alcohol in Perfume
The concentration of alcohol in perfumes varies significantly depending on the type of fragrance:
Eau de Cologne: Contains about 2-5% aromatic oils with a high alcohol content, resulting in a very fresh, quickly evaporating scent.
Eau de Toilette: Typically 5-15% aromatic oils, offering a moderate evaporation rate and good fragrance longevity.
Eau de Parfum: Ranges from 15-20% aromatic oils, with alcohol ensuring balanced evaporation and substantial fragrance longevity.
Parfum or Perfume Extract: Contains 20-40% aromatic oils with lower alcohol content, producing an intense, long-lasting scent with minimal alcohol evaporation.
We have an article that covers these categories in depth here.
Summary
Bottom line, alcohol is important for scent delivery, evaporation dynamics, and skin interaction. All of these factors make it integral to the craft and experience of perfume wearing. Without it, many perfumes today would not exist.


